Description
Medical low-density polyethylene bags are pharmaceutical packaging materials that are produced in a clean environment using polyethylene particles as raw materials through processes such as film blowing, bag making, heat sealing, and vacuum packaging, and can directly contact drugs. The entire product process is completed in a D-level or C-level clean environment to meet the cleanliness requirements of different pharmaceutical customer products.
Performance advantages
The raw materials meet the standard requirements for food and pharmaceutical grade indicators
Thin film with high strength and puncture resistance, can be customized for products with an inner thickness of 30 threads
Good compatibility with the vast majority of APIs and good product stability
The finished product has low particulate matter and meets high cleanliness requirements
The product is easy to heat seal, suitable for customers to store the product in a sealed manner
SFDAApproved drug packaging material registration certificate and CDE filing number
Each batch of products is accompanied by a factory inspection report as required
Ordering information
|
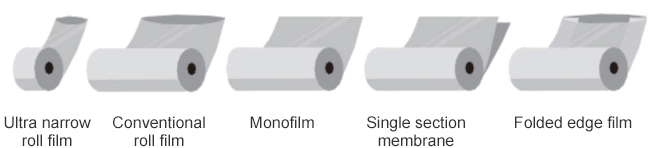
film |
 |
|
Bag |
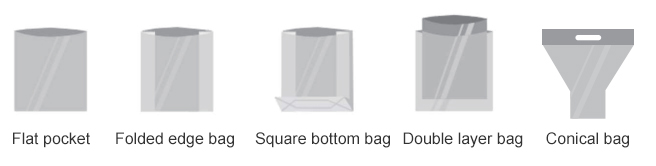 |
|
Material quality |
LDPE/LLDPE/HDPE |
|
Quality standard |
YBB00072005-2015 Q/320412 DRE001-2021 EP USP Customer customization |
|
Functionality |
High transparency, easy to tear dashed lines, anti-static, light avoidance, low particle count, color customization, etc |
|
Package |
Vacuum packaging, double-layer packaging, cardboard boxes, kraft paper bags or according to customer needs. |
